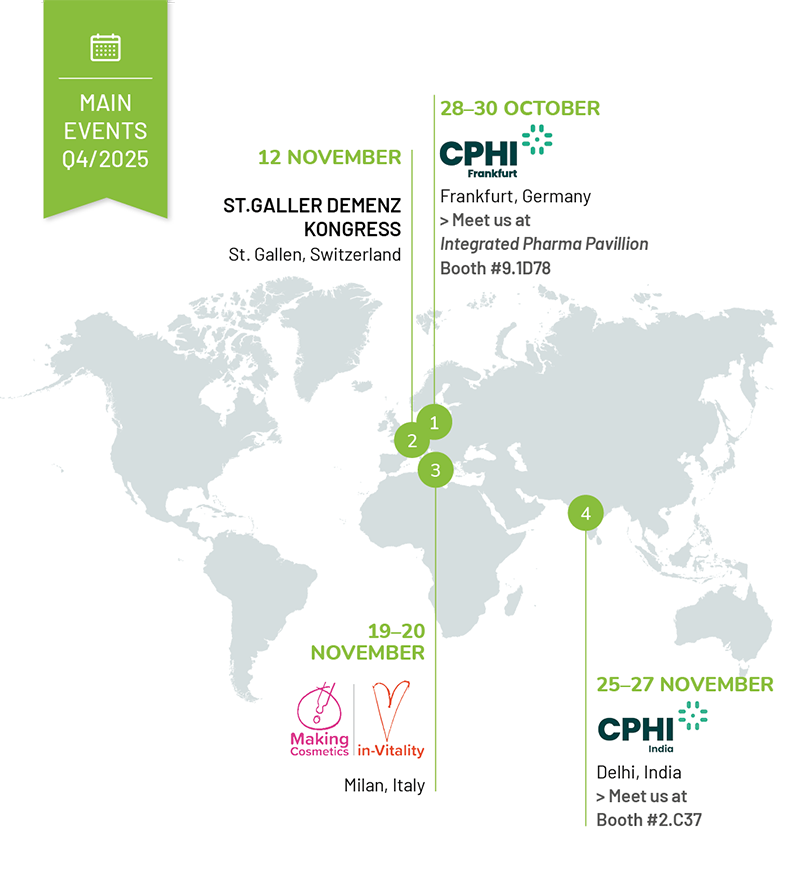
OCTOBER

28–30 October
CPHI Worldwide
Frankfurt, Germany
Messe Frankfurt
> Meet us at Integrated Pharma Pavillion | Booth 9.1D78